CRISPR based medicine has rapidly become one of the most disruptive forces in modern gene therapy, transforming how researchers, clinicians, investors, and regulators think about disease treatment and intellectual property. Nowhere is this more visible than in blood disorders, where gene therapy approaches targeting sickle cell disease, beta-thalassemia, and haemophilia have moved from experimental promise to clinical reality. At the same time, gene therapy patent trends are reshaping the global innovation economy, as universities, biotech companies, and pharmaceutical giants compete to define ownership over foundational technologies, delivery platforms, and clinical applications.
What began as a biological curiosity has evolved into a multi-billion-dollar ecosystem involving gene therapy, venture capital, multinational licensing agreements, and high-stakes litigation. Understanding the future of CRISPR based medicine therefore requires two lenses: the scientific evolution of gene therapy itself, and the parallel evolution of gene therapy patent trends that determine who ultimately controls access to these technologies.
How CRISPR Based Medicine Is Redefining Gene Therapy
The origins of CRISPR technology lie in a natural immune defense system found in bacteria and archaea. These microorganisms capture fragments of viral DNA and store them in their own genome, enabling a memory-based defense against future infections. Scientists later discovered that this system, known as CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats), could be repurposed as a programmable platform for gene editing.
Through the combination of CRISPR sequences and Cas (CRISPR-associated) proteins, researchers developed CRISPR gene editing tools capable of cutting DNA at precise locations. This breakthrough transformed the broader field of gene therapy, which had long struggled with issues of accuracy, safety, and scalability. Unlike earlier gene therapies that relied heavily on viral vectors with limited precision, CRISPR gene editing allows targeted correction of disease-causing mutations.
This capability fundamentally changed how gene therapy could be applied. Rather than treating symptoms, CRISPR-based medicine made it possible to target the root genetic causes of disease. For monogenic disorders: conditions driven by a single mutation, this opened a pathway toward durable, potentially curative gene therapies.
CRISPR Based Medicine as a Molecular Precision Platform
CRISPR gene editing supports multiple therapeutic strategies within modern gene therapy programs:
This versatility explains why medicine based on CRISPR has become central to next-generation gene therapy pipelines worldwide. It also explains why gene therapy patent trends increasingly focus not only on applications, but on refinements to CRISPR gene editing systems themselves.
Blood Disorders and the Rise of CRISPR Gene Therapy
Blood disorders have emerged as the most clinically advanced domain for CRISPR based medicine. The biology of blood offers a unique advantage for gene therapy because hematopoietic stem cells can be edited outside the body and reinfused, enabling durable therapeutic effects. These hematopoietic stem populations sit at the core of many successful gene therapy strategies.
CRISPR-based gene therapies are actively being developed for:
The approval of Casgevy (exagamglogene autotemcel) in late 2023 marked a defining moment in the history of gene therapy. Developed through collaboration between Vertex Pharmaceuticals and CRISPR Therapeutics, Casgevy became the first approved CRISPR-based gene therapy for sickle cell disease and transfusion-dependent beta-thalassemia. This milestone validated decades of research in gene therapy and confirmed the commercial viability of CRISPR gene editing in real patients.
Clinically, Casgevy works by editing hematopoietic stem cells ex vivo to increase fetal hemoglobin production. These edited hematopoietic stem cells are reinfused, leading to long-term therapeutic benefits. The success of this approach has reshaped both clinical confidence in gene therapy and strategic behavior around patents in gene therapy globally.
Delivery Technologies and the Evolution of Therapy Patents
As CRISPR based medicine matures, innovation has shifted from whether gene therapy works to how it can be delivered safely and efficiently. Delivery is now the central bottleneck in next-generation gene therapies, and consequently one of the most heavily patented areas within the ecosystem.
Modern patents on therapy increasingly focus on:
This shift is clearly visible across gene therapy patent trends. Early filings emphasized CRISPR gene editing tools themselves; newer filings emphasize delivery architectures, manufacturing improvements, and safety optimizations. In effect, the competitive frontier of gene therapy has moved from invention to execution.
Mapping the Global Patent Landscape for CRISPR Gene Therapy
The patent landscape surrounding CRISPR technologies reflects the scale of commercial interest in gene therapy. Globally, more than 11,000 patent applications reference CRISPR systems across all application areas. Within the narrower domain of CRISPR-based blood disorder treatment, the active patent landscape comprises approximately 1,700 patent families.
However, only a fraction of these assets has matured into granted patents, while the vast majority remain under examination. This indicates that the field is still structurally unsettled, with claim boundaries, ownership strength, and freedom-to-operate positions continuing to evolve.
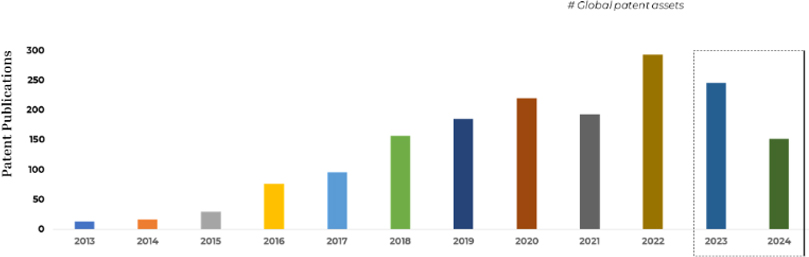
The figure above represents Overall Filing Trends over the years
From an IP strategy standpoint, this skew toward pending applications suggests intensifying competition, high prosecution risk, and future consolidation of rights as patents begin to grant. It also means that portfolio quality and prosecution strategy matter more than filing volume, particularly for companies approaching clinical or commercial milestones.
In essence, the landscape reflects a technology that has moved beyond discovery, but whose legal and commercial contours are still being defined.
More than 90% of all filings occurred after 2016, reflecting how quickly the field matured once CRISPR proved clinically viable.
Geography of Innovation: Where the IP Is Concentrated
Patent protection in this domain is highly strategic and geographically uneven.
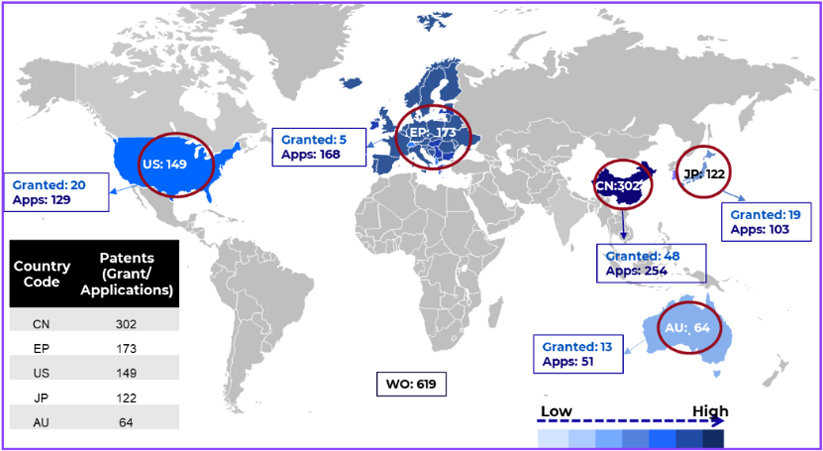
Figure represents jurisdiction wise filling in the patent filling in use of CRISPR/CAS System for Treatment of Blood Disorder
China’s dominance is further reinforced by institutional assignees such as Shanghai University and BioRay Lab Inc. Japan accounts for 7.2% of filings, while Australia represents 3.8%.
Notably, outside China and Japan, most Asian countries remain underrepresented, creating visible whitespace opportunities in jurisdictions such as India and parts of Southeast Asia.
Gene Therapy Patent Trends (2021–2025): A Maturing Innovation Cycle
The trajectory of gene therapy patent trends over the last five years reveals a clear evolution in how organizations approach CRISPR based medicine.
Gene therapy patent trends 2021
In 2021, the focus of gene therapy filings remained exploratory. Many applications centered on platform technologies, guide RNA architectures, and early delivery concepts. Universities and early-stage biotech firms dominated activity, reflecting a field transitioning from discovery science into translational gene therapy development.
Gene therapy patent trends 2022
By 2022, clinical confidence increased sharply. Late-stage programs in sickle cell disease and oncology accelerated portfolio building across the sector. The patent trends of 2022 showed strong growth in method-of-treatment claims, manufacturing innovations, and delivery-focused filings. Organizations increasingly treated gene therapy as a commercial asset class rather than speculative technology.
Gene therapy patent trends 2023
The approval of Casgevy fundamentally altered the ecosystem. These patent trends 2023 reflect a shift away from sheer filing volume toward portfolio refinement. Companies focused on continuation filings, strategic claim narrowing, and defensibility, signaling that gene therapy had entered a regulated, revenue-generating phase.
2024–2025: Consolidation and Portfolio Quality
By 2024 and 2025, these patent trends increasingly emphasized quality over quantity. Narrow, high-value claims aligned with regulatory approvals became the norm. Investors and partners began scrutinizing portfolio strength more rigorously, creating a separation between leaders with defensible positions and followers with weaker patents.
Who Controls Innovation in CRISPR Based Medicine?
The ecosystem surrounding CRISPR based medicine is shaped by both academic and commercial leadership. Institutions such as the Broad Institute, MIT, Harvard, Stanford, and the University of California continue to hold foundational IP that underpins much of modern gene therapy development. These academic portfolios explain why licensing remains central to commercial gene therapy strategies.
On the commercial side, companies such as CRISPR Therapeutics (who has in particular, emerged as a central player linking scientific leadership with regulatory success in gene therapies), Editas Medicine, Vertex Pharmaceuticals, and Novartis are building highly focused portfolios aligned with specific clinical programs.
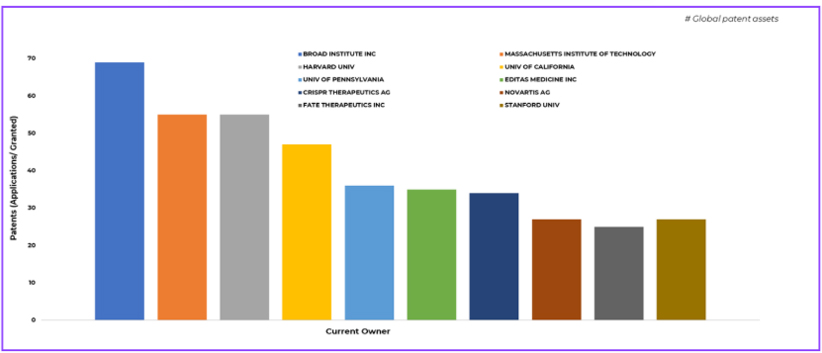
The figure highlights the major patent holders driving innovation in CRISPR-based treatment of blood diseases.
This competitive structure ensures that innovation in gene therapy remains dynamic. New entrants such as Sana Biotechnology and emerging platform companies continue to reshape gene therapy patent trends, demonstrating that leadership is not yet locked in.
Litigation, Competition, and the Economics of Gene Therapy Patents
The enormous value associated with CRISPR based medicine has made litigation inevitable. Foundational disputes between the Broad Institute and the University of California illustrate how ownership of core CRISPR gene editing inventions continues to influence commercial positioning across the entire gene therapy sector.
Early filings by companies such as Editas Medicine helped define first-generation applications in sickle cell disease and beta-thalassemia. More recent filings increasingly emphasize immune evasion, cell engineering, and next-generation delivery, demonstrating how gene therapy innovation has moved from feasibility to optimization.
Emerging inventions, including those involving compact Cas variants and base-editing platforms, signal a future where gene editing becomes even more precise, safer, and scalable. These advances will drive the next wave of gene therapy patent trends globally.
CRISPR Based Medicine – Cost, Reviews, and Market Reality
One of the most frequently discussed aspects of CRISPR based medicine is pricing. The cost of CRISPR based medicine for first-generation therapies such as Casgevy has been reported in the range of USD 2–3 million per patient. This reflects complex manufacturing, individualized cell processing, and years of R&D investment.
Unsurprisingly, this pricing has influenced both public perception and CRISPR based medicine reviews across healthcare systems, media outlets, and patient advocacy groups. While clinical outcomes are widely regarded as transformative, concerns remain around access, reimbursement models, and long-term scalability of gene therapy.
These economic pressures are already shaping innovation priorities. Newer gene therapies increasingly aim to reduce manufacturing complexity, simplify delivery, and enable more scalable treatment models. Future patents are therefore likely to focus not only on biology, but on operational efficiency.
CRISPR Clinical Trials and Expanding Therapeutic Horizons
Beyond approved products, the ecosystem of clinical trials in CRISPR continues to expand rapidly. As of 2025, there are more than 150 active CRISPR trials globally, covering blood disorders, oncology, ophthalmology, and metabolic diseases. These programs collectively represent the next wave of gene therapy innovation.
Public interest in these trials has generated recurring questions, including:
When will CRISPR be available to the public?
Approved therapies such as Casgevy are already available in select healthcare systems, but broader public access will take time due to infrastructure, cost, and regulatory factors.
When will CRISPR be available for HIV?
Research into CRISPR for HIV remains active, but clinical applications are still early. While experimental programs exist, CRISPR HIV human trials have not yet produced approved therapeutic products, and safety remains a key research focus.
These realities highlight both the promise and the complexity of translating CRISPR gene editing from laboratory success into scalable global gene therapy.
The Future of Gene Therapy and Patent Strategy
Looking forward, the trajectory of CRISPR-based medicine suggests continued expansion of both scientific capability and commercial competition. The next decade of gene therapy is likely to be shaped by:
For stakeholders across biotech, healthcare, investment, and policy, understanding gene therapy patent trends will become as important as understanding clinical data. Ownership of IP will increasingly determine who can develop, distribute, and scale future gene therapies worldwide.
Why CRISPR Based Medicine Represents a Structural Shift in Healthcare
CRISPR based medicine is not simply another category within gene therapy. It represents a structural redefinition of how medicine itself is conceptualized. Traditional pharmaceuticals are built around chronic management: reduce symptoms, slow progression, and mitigate damage. In contrast, CRISPR gene editing positions medicine as a one-time molecular intervention capable of delivering long-term or permanent therapeutic impact.
This is precisely why the patent landscape around CRISPR based medicine is expanding so rapidly. The economic logic of curative therapies differs radically from the economics of maintenance drugs. When a therapy potentially cures sickle cell disease, beta-thalassemia, or inherited immunodeficiencies, its value proposition is no longer measured in incremental improvement but in lifelong health transformation. That shift is clearly reflected in gene therapy patent trends over the past decade, where filings increasingly focus on durability, delivery optimization, and next-generation editing systems rather than basic feasibility.
Reinforcing the Clinical Reality: Clinical Trials and Commercial Validation
One of the most important signals visible in these clinical trials is that these are no longer experimental proofs of concept. The regulatory approval of Casgevy established a precedent that now shapes both investment behavior and patent filing strategy globally. Companies are no longer filing speculative applications around theoretical gene editing methods; instead, they are building defensible portfolios around delivery platforms, ex vivo editing workflows, hematopoietic stem cell modification, and scalable manufacturing techniques.
This evolution mirrors broader gene therapy patent trends seen in adjacent modalities such as CAR-T therapies and RNA-based therapeutics. As therapies move closer to commercialization, patent claims become narrower, more strategic, and more tightly aligned with clinical use cases. This is why the current patent data, where most applications remain pending, is strategically significant. It signals that claim boundaries are still being negotiated, prosecution strategies still evolving, and competitive positioning still fluid.
Economic and Access Questions: Medicine Cost and Market Implications
Another emerging layer of discourse around CRISPR based medicine relates to pricing and accessibility. While this article focuses on patent landscapes and innovation dynamics, it is impossible to ignore that the medicine’s cost will play a decisive role in shaping adoption.
Early gene therapies and cell-based therapies have already demonstrated six-figure and even seven-figure pricing models. This economic reality directly influences patent strategy. Strong IP portfolios are not merely defensive tools; they are mechanisms through which companies justify reimbursement negotiations, licensing frameworks, and long-term commercial viability. As more CRISPR therapies reach market approval, the interplay between gene therapy patents, pricing strategies, and healthcare policy will become increasingly central.
Future-Facing Signals: Where the Next Wave of Patents Is Likely to Emerge
Based on both the quantitative data and qualitative trajectory described throughout this article, several areas are likely to dominate future patent activity:
These directions align with broader biotechnology investment flows and reinforce why CRISPR based therapeutics is no longer a niche research field but a central pillar of next-generation medicine.
Conclusion
CRISPR based medicine has moved decisively from scientific concept to clinical and commercial reality. The evolution of gene therapy for blood disorders demonstrates both the therapeutic power of CRISPR gene editing and the strategic importance of intellectual property in shaping access to innovation. At the same time, gene therapy patent trends reveal a rapidly maturing ecosystem where portfolio quality, defensibility, and delivery innovation increasingly define competitive advantage.
For patients, this transformation offers unprecedented hope. For innovators, it offers extraordinary opportunities. And for the global healthcare system, it poses profound questions about equity, access, and governance. In this new era, the future of gene therapy is being written not only in laboratories and hospitals, but in patent offices around the world.
Download Report

Download Report